Patient Survey Results
The first survey was to determine whether there is sufficient patient need among those that have a qualifying diagnosis to receive medical cannabis by surveying patients directly, and by reviewing data from the Public Employees Insurance Agency (PEIA) and the state Medicaid program. Among the qualifying conditions that allow a patient to receive a recommendation for marijuana under West Virginia’s law are chronic pain, post-traumatic stress disorder (PTSD), neuropathy, and cancer. In the patient survey, 2,120 patient responders reported that they suffered from chronic pain, 1,579 reported that they had PTSD, 807 reported neuropathies, and 694 reported cancer. In reviewing PEIA data for two years, there were 8,632 claims submitted for neuropathies, 8,378 submitted claims for cancer, 6,561 submitted claims for chronic pain and 6,289 submitted claims for PTSD. Among Medicaid recipients for a similar 18-month period, 55,666 claims were submitted for patients with cancer, 25,101 claims were submitted for patients with chronic pain, and 10,692 claims were submitted for patients with PTSD. Based upon these informal surveys and data, there is a strong indication that sufficient patients with a qualifying medical condition exist to warrant a medical cannabis program.
Physician Survey Results
Of the 1,455 physicians participating in an online survey, 82 percent expressed an interest in participating in the medical cannabis program. Obviously, this percentage may exaggerate the percentage of physicians that will ultimately participate in the medical cannabis program since it is more likely that those responding would have such an interest, but the significant number of responses indicates there will be sufficient interest in the physician community to serve those with a serious medical condition that are eligible and will likely benefit from medical cannabis. The patient survey may also be interpreted as self-selecting or biased in favor of those interested in medical cannabis.
Proposed Rules
Also at the December 14, 2017 meeting, the Bureau for Public health announced prosed rules to govern the implementation of new medical cannabis programs and licenses under the West Virginia Medical Cannabis Act:
- Proposed rules governing the general licensing of medical cannabis regulated parties (proposed W.Va. C.S.R. §64-109-1, et seq.).
- Proposed rules governing the licensing and regulations of growers and processors (proposed W.Va. C.S.R. §64-110-1, et seq.).
- Proposed rules governing the licensing and regulations of laboratories for medical cannabis programs (proposed W.Va. C.S.R. §64-111-1, et seq.).
- Proposed rules governing the licensing and regulations of dispensaries (proposed W.Va. C.S.R. §64-112-1, et seq.).
- Proposed rules regarding a safe harbor letter relating to the use of medical cannabis by the terminally ill if purchased in other states than West Virginia (proposed W.Va. C.S.R. §64-113-1, et seq.).
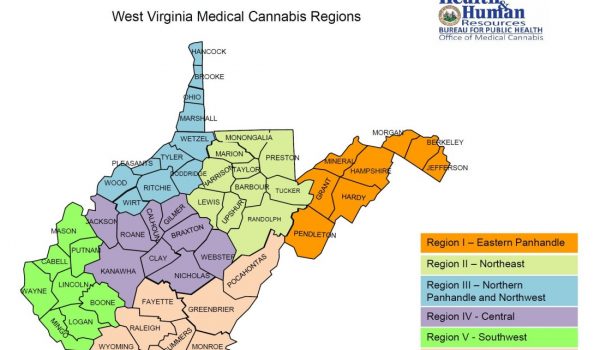
- A proposed regional map which establishes six (6) regions throughout West Virginia to govern the allocation of licenses to the foregoing prospective licensees.
Copies of the above rules and the map can be obtained on the Office of Medical Cannabis website.
The West Virginia Medical Cannabis Advisory Board is receiving comments on the proposed rules to enable all affected to comment prior to the rules becoming effective. The comment period ends on January 12, 2018, at 11:59 p.m.
Comments on the proposed rules may be submitted by email to: Brian.J.Skinner@WV.gov or by U.S. Mail to: Brian J. Skinner, West Virginia Bureau for Public Health, 350 Capitol Street, Room 702, Charleston, WV 25301.
If you require further information regarding the proposed rules or need assistance in submitting comments, please contact Charles M. Johnson at cmjohnson@fbtlaw.com.